| Home | Phyllosoma | Culturing | Jellies | Molecular Methods | Publications |
|---|
Habitat restoration through understanding the phyllosphere:
plant surfaces, their microbes, and the animals that eat those microbes

Microbial diet of endangered tree snails across phyllospheres
Snails were once numerous across the Hawaiian archipelago and filled numerous ecological roles across multiple habitats. However, mass extinctions have occurred due to predator introductions and habitat loss, and therefore human intervention is probably the only way that many of these species will avoid this trend of decline.

We used high-throughput DNA sequencing to determine variance in the diet of a federally endangered tree snail, Achatinella mustelina, which is endemic to the island of Oahu. These snails dwell on trees and graze microbes that grow on leaf surfaces - technically referred to as the "phyllosphere". The microbiology of the diet of these animals could be crucial to understanding how they can be successfully relocated into predator-proof enclosures or ex situ culture facilities.
This work was done in the botany department of the University of Hawai’i at Manoa for Professor Anthony Amend. The Amend lab is devoted to the study of fungal ecology in a variety of terrestrial and aquatic contexts.
Diet across the range of Achatinella mustelina, Achatinella sowerbyana and Achatinella lila.
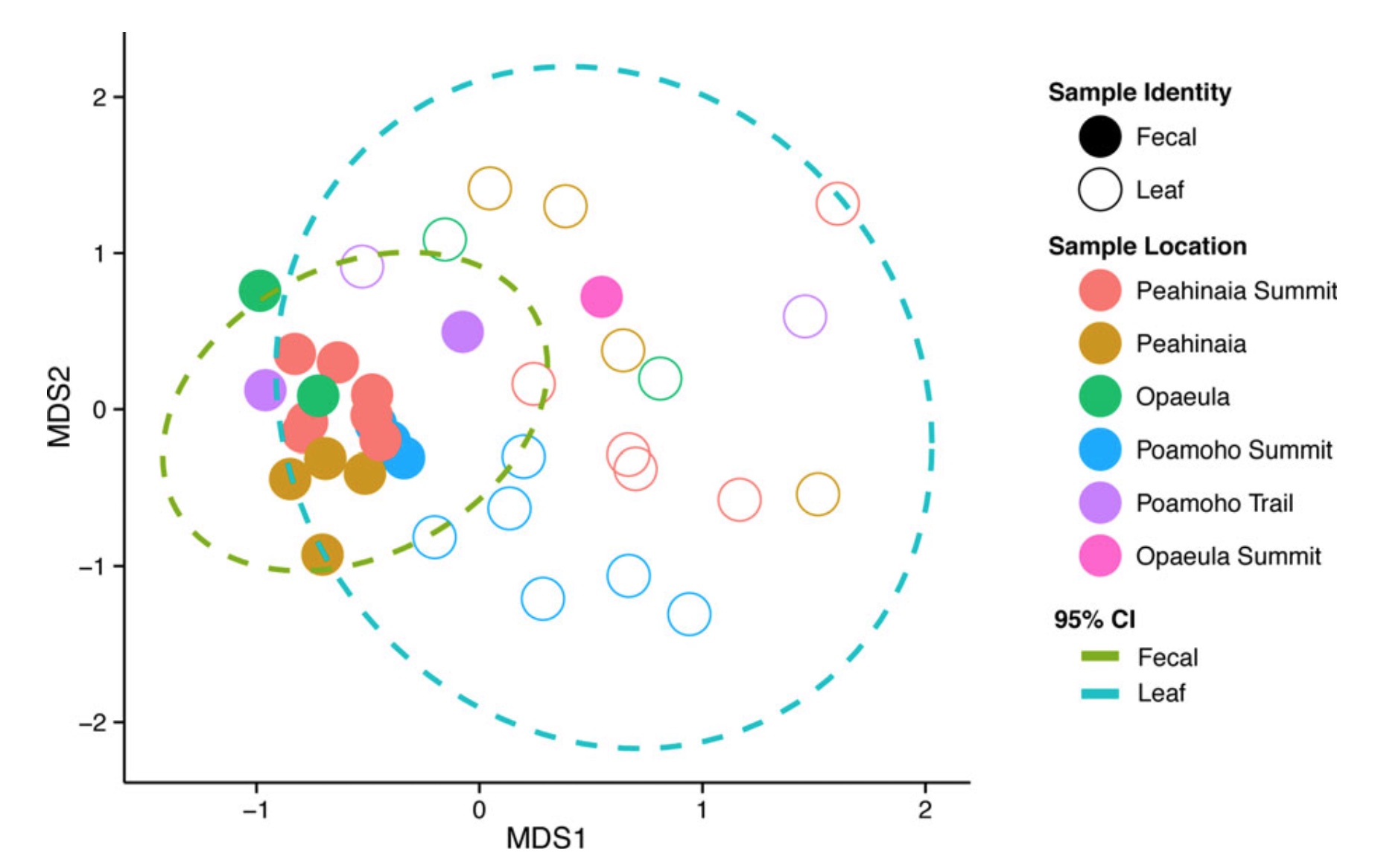
A fundamental question about a species' feeding behavior is “how does its diet vary across its geographic range”? This is a crucial thing to understand if a threatened species is to be managed by relocation into predator free enclosures or artificial habitats. When conservationists began to rescue snails by translocating them to artificial ex situ facilities they did some of this fundamental ecological research: they isolated strains of fungi from the snails’ host plant and conducted trials into whether snails would feed and grow on particular strains. Advances in DNA sequencing technology over the last few years mean that we no longer need to use a time consuming approach of testing if a fungus is snail-food by isolating it from the snails' habitat and then conducting feeding trials (although there are some benefits to this approach). We can instead sequence eDNA directly from the snails feces to discover what was in their gut, and also sequence eDNA from the snails habitat to see what food was available to them.
We looked at the diet of four populations of Achatinella mustelina on the Waianae mountain range (from within and outside of predator proof enclosures). Dr Melissa Price conducted a separate study where she collected feces from the snails Achatinella sowerbyana and Achatinella lila and leaf microbiomes from the windward slopes of the Ko’olau mountain range. Finding remnant populations of these snails is extremely difficult and our work relied heavily on the efforts and expertise of conservationists from the OANRP, PCSU and US Fish and Wildlife Service.
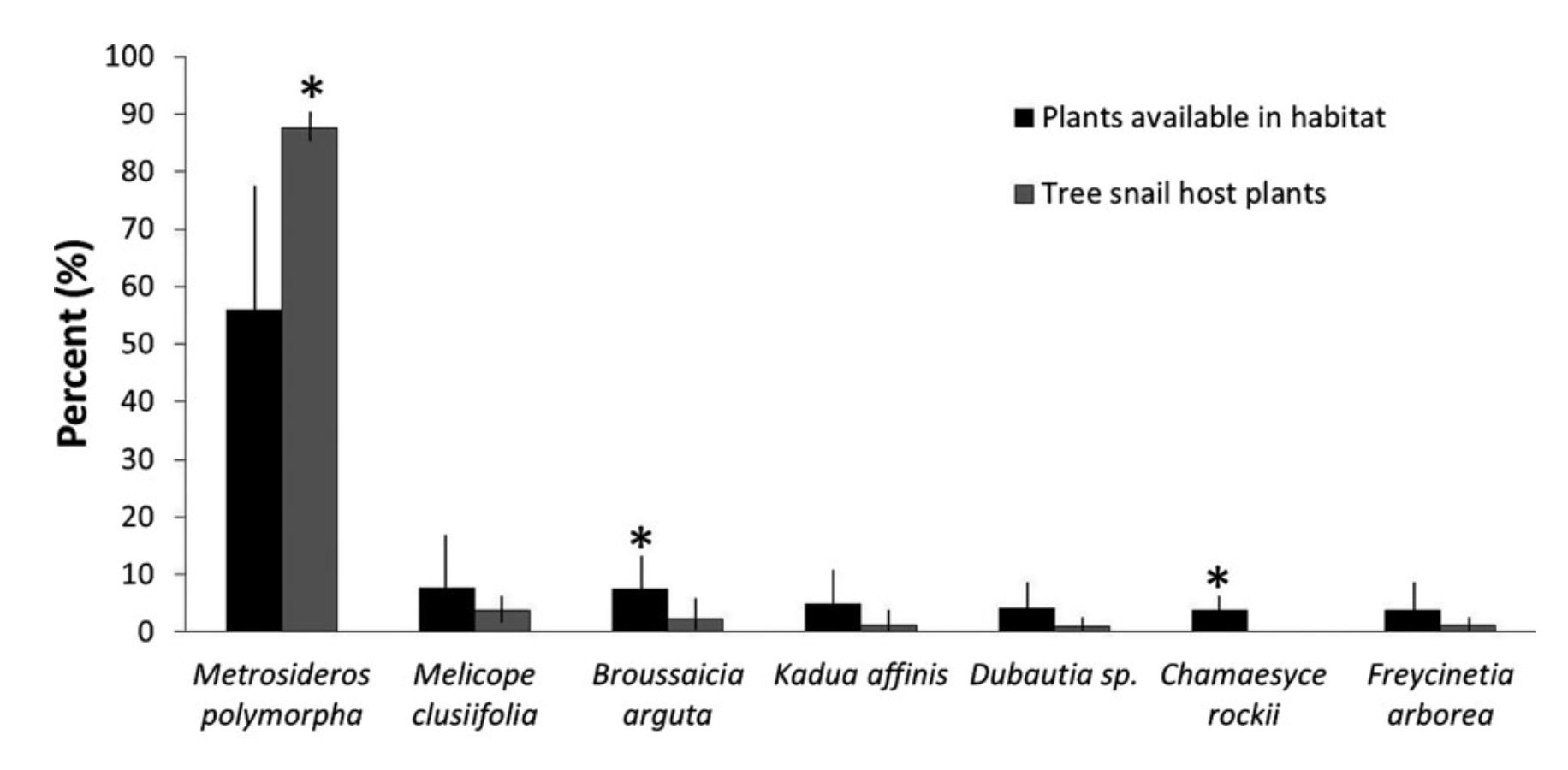
Comparisons were made between the diversity of fungal species on leaves (what the snails might eat) and the diversity of fungi in the snails feces (what they have actually eaten). Similar fungi occurred in both leaves and feces, but their abundances varied. In the Ko’olau Ranges the diversity of fungi on leaves is considerably more divergent than in their feces, suggesting that A. sowerbyana and A. lila could be selectively consuming or digesting fungi. Melissa also found that snails in the Ko’olau mountain range tended to graze fungi off leaves of a particular tree, the ʻōhiʻa lehua (Metrosideros spp.).

Mesocosm study - testing snail modification the phyllosphere
Animals that feed on fungi can shape the fungal community in a several ways. For example, by grazing away biomass they release minor members of the community from competitors. They can also influence the community by dispersing spores and vegetative fragments of their food after it passes through them.
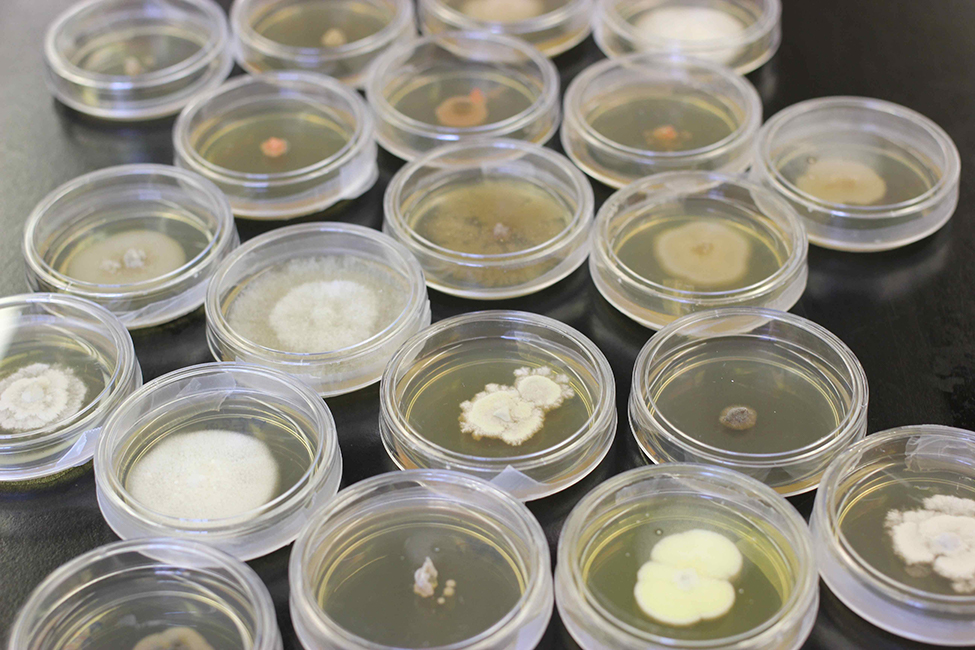
There is a marsh snail (named Littoraria irrorata) that “farms” the fungus that it prefers to eat. This marsh snail poops on wounded leaves, and the fungal spores or propagules in their poop grow in the wound and can be eaten later. Because this is a well-known example, and because Hawaiian Achatinella snails poop on the leaves they feed from, many biologists would ask me if I thought the Hawaiian snails were farming the fungus that they ate. We conducted an experiment to test this. The idea seemed plausible, especially because Kapono and I, and also Brenden Holland and his team, found that diverse fungal species could be isolated from poop and cultured on different agar plates.
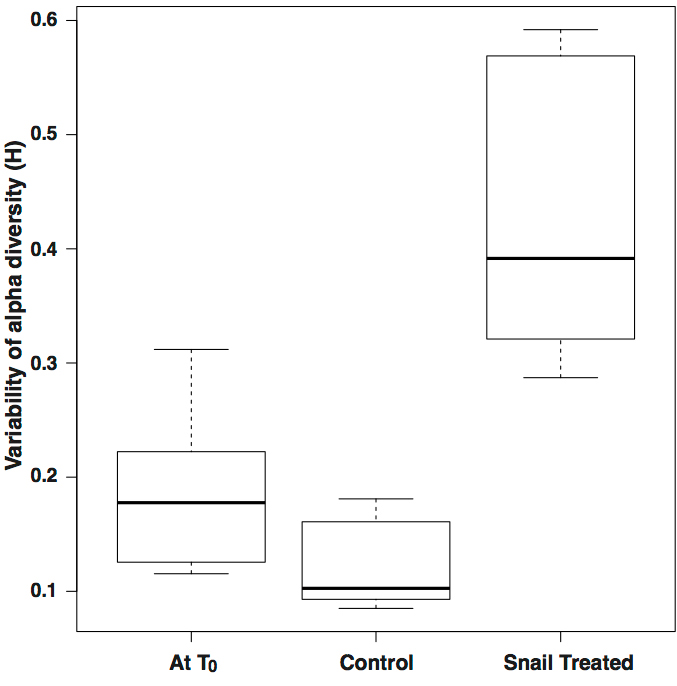
Our experiment consisted of pairs of mesh bags ("mesocosms") that we placed over tree branches. At the start of the experiment we took wild Auriculella ambusta snails and looked at what food species were in their poop using eDNA. We then put snails into one of the mesocosms (but not the matched pair, which was a control). We then visited the study site over six weeks to see if the bags with snails would become colonized with the species of fungus that the snails fed on and pooped out. They didn’t. However, the fungi growing in enclosures with snails did differ from the control enclosures without snails; the snails created more variability in the biodiversity of the fungus. We thought that this was consistent with grazing facilitating the invasion of environmental fungus into the leaf community. With help from Tina Carvalho from the University of Hawai’i Biological Electron Microscopy Facility we compared microbe abundances between leaves from grazed and control enclosures. Our measurements were consistent with the hypothesis that snails were clearing large regions of fungi and therefore facilitating invasion.

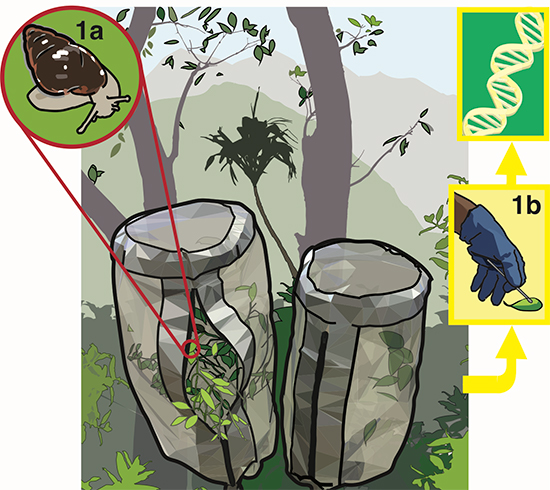
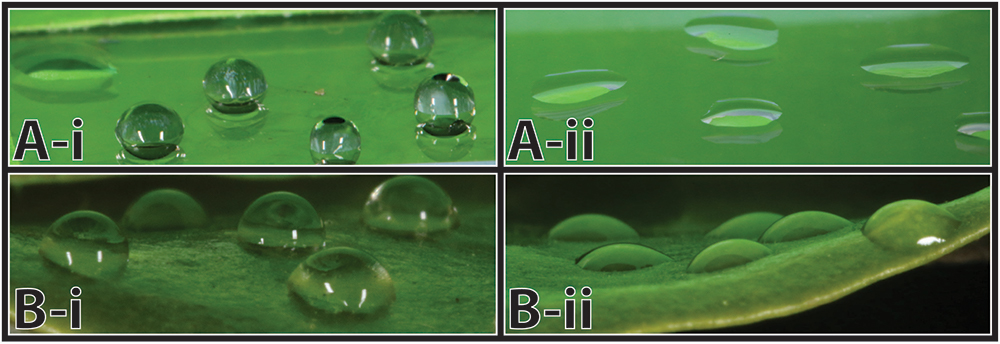
On a tangent, I did get interested in why viable fungi in the snails' poop weren’t growing in the mesocosms. This seemed odd because leaf surfaces are very nutrient poor, so I thought it was intuitive that a poop should be one of the best fertilised places for fungi to grow – shouldn’t it? Snail feces are compacted with mucus, and although pedal mucus and fecal mucus differ a little, I thought it worth looking at pedal mucus as a proxy (apologies if too much mention of feces and mucus is making your stomach turn). I found that Auriculella pedal mucus is very hydrophobic (repels water), and a lack of water can be a strong factor in preventing microbial growth. I thought that this was pretty interesting, especially because I could only find one other reference to snail mucus – and for that species it was hydrophilic, the complete opposite! So I started a project, that I am yet to complete, to see how snail mucus hydrophobicity varies with snail phylogeny and ecology.
Take home message: These snails certainly modify fungal communities by grazing, which allows new fungal species to invade the leaf surface and give it a local “flavor”. However, they do not seem to disperse the fungus after they eat it.
Read the Publications
O’Rorke, R., Tooman, L., Gaughen, K., Holland, B.S. and Amend, A.S. (2017) Not just browsing: an animal that grazes phyllosphere microbes facilitates community heterogeneity. The ISME Journal 11, 1788–1798
O’Rorke, R., Cobian, G.M., Holland, B.S., Price, M.R., Costello, V., Amend, A.S. (2015) Dining local: the microbial diet of a snail that grazes microbial communities is geographically structured. Environmental Microbiology 17 (5), 1753-1764
Price, M.R., O’Rorke, R., Hadfield, M.G., Amend, A.S. (2017) Diet Selection at Three Spatial Scales: Implications for Conservation of an Endangered Hawaiian Tree Snail. Biotropica. 49, 130–136.
O’Rorke, R., Holland, B.S., Cobian, G.M., Gaughen, K., Amend, A.S. (2016) Dietary preferences of Hawaiian tree snails to inform culture for conservation Biological Conservation 198, 177–182.
| Home | Phyllosoma | Culturing | Jellies | Molecular Methods | Publications |
|---|